What is Ocean Acidification?
Oceans provide a great service to the planet by slowing down the accumulation of CO2 in the atmosphere, which is the major cause of global warming. Unfortunately, the additional CO2 is changing the fundamental chemistry of the oceans. CO2 dissolves in surface water to form carbonic acid, which upon dissociation results in a decrease in pH and the concentration of the carbonate ion, a building block of calcium carbonate (CaCO3) shells and skeletons (Bedford Institute of Oceanography, Canada, 2019).

Ocean acidification (OA) basically refers to the decrease in pH and carbonate ion concentration due to the increasing anthropogenic CO2 in the ocean. Annually, about one third of the carbon dioxide (CO2) in fossil fuel emissions dissolves in ocean surface waters, forming carbonic acid and increasing ocean acidity. Over the next century or so, it is forecasted that acidification will be intensified near the surface where much of the marine life resides that humans depend upon for food and trade.
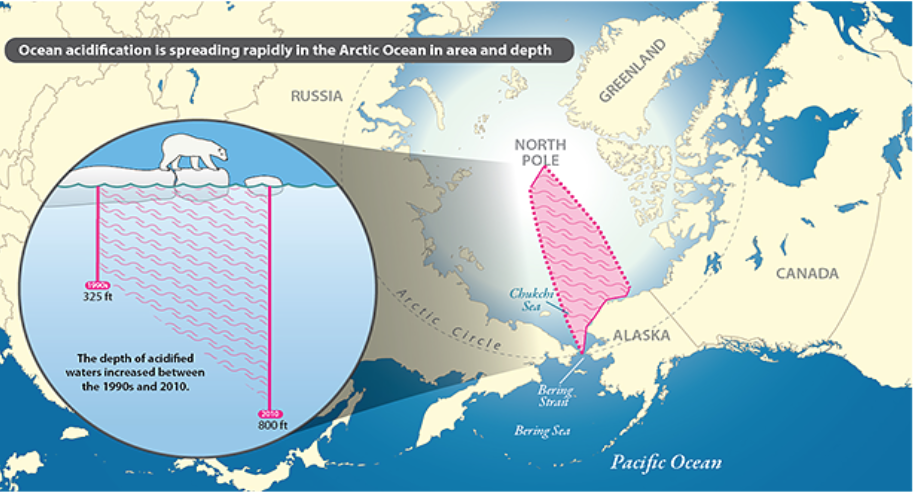
The ocean surface is becoming more acidic with increasing atmospheric CO2, and acidity has increased by about 30% since the beginning of the industrial revolution (Government of Canada, 2012). The problem is that the majority of the CO2 has been absorbed by the cold polar waters because CO2 is more soluble in cold water.
Recently, melting sea ice has allowed for more Pacific water to flow into the Arctic Ocean and build up there. Pacific Ocean water is already high in carbon dioxide. As the ocean mass moves, it absorbs additional carbon dioxide from decomposing organic matter in the water and sediments, increasing the water’s overall acidity (Allen, 2019).
The melting and retreating of Arctic sea ice in the summer months has allowed Pacific winter water to move further north. In the image below, ocean acidification is spreading in area and depth in the western Arctic Ocean.
The Importance of the Arctic Ocean
Canada’s oceans are distinctly influential in acidification. In the Pacific, wind-driven surges bring the intermediate depth water, corrosive to organisms with CaCO3 shells and skeletons, to the surface. Although, it is a natural phenomenon and occurs seasonally, this intermediate water is inherently more concentrated with CO2 (due to microbial respiration). Furthermore, addition of anthropogenic CO2 lowers the pH to a critical level.
It was in the Arctic that the first observations of corrosive surface ocean waters were reported. The Arctic Ocean receives a large amount of fresh water from rivers, seasonal ice melt, and glacial meltwater. This fresh water has little buffer capacity against acidity and reduces the pH of Arctic waters. Decreased ice cover increases absorption of atmospheric CO2, further accelerating Arctic acidification.
In the Atlantic, the effects of corrosive Arctic water, freshwater input from the St. Lawrence River, hypoxic (low oxygen) water in the Gulf of St. Lawrence, and high rates of anthropogenic CO2 absorption in the deep convection region of the Labrador Sea act as enhancers in ocean acidification (Bedford Institute of Oceanography, Canada, 2019). The result is increasingly acidic oceans.
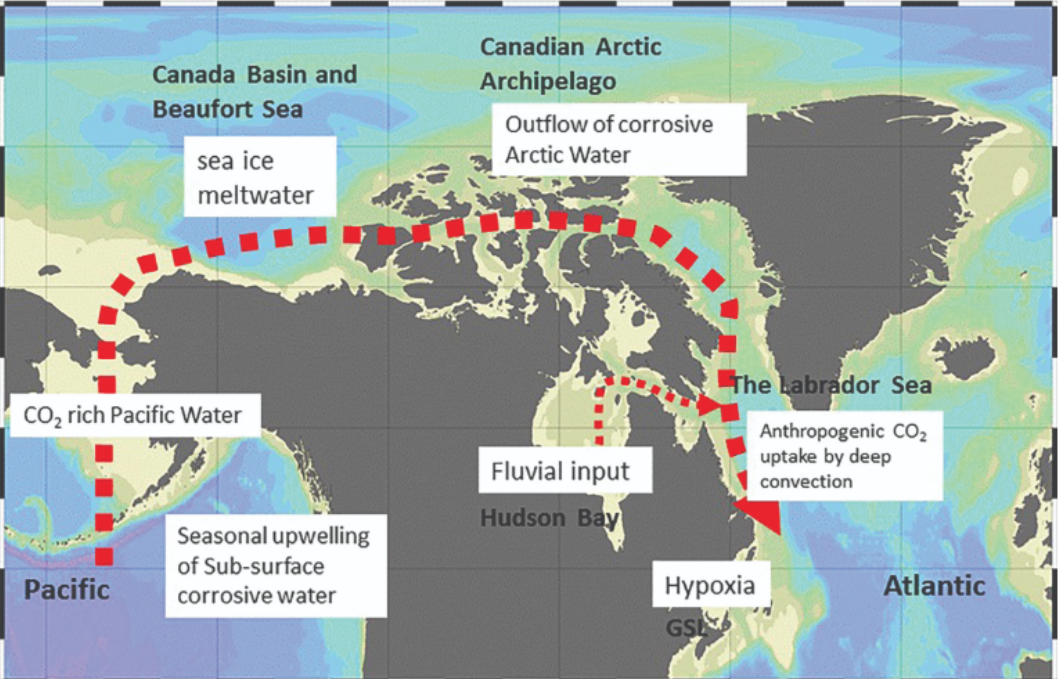
This is why the cold waters of Canada are particularly vulnerable to ocean acidification. The polar regions are also susceptible and vulnerable to ocean acidification due to the chemical composition of the oceans.
Why is this a problem for marine and human life?
Rising acidity eats up the minerals vital to shell-building creatures, as well as posing other dangers to marine life. The growth rate and mortality of marine life are negatively affected. Resultantly, ocean acidification alters the structure and function of its marine ecosystems. Hence, fishing industries, subsistence fisheries by Indigenous communities, and tourism will be directly influenced. The results of these changes will threaten economies, especially those directly connected with fisheries, culture, and subsistence of Indigenous peoples. (Bedford Institute of Oceanography, Canada, 2019).
______
Bibliography
Allen, M. (2019, march 29). Research shows ocean acidification is spreading rapidly in the Arctic. Retrieved from climate.gov: https://www.climate.gov/news-features/features/research-shows-ocean-acidification-spreading-rapidly-arctic
Bedford Institute of Oceanography, Canada. (2019). Ocean Acidification in Canadian Waters. In K. Azetsu-Scott, The Future of Ocean Governance and Capacity Development (pp. 227-231). Brill | Nijhoff.
Government of Canada. (2012). What is Ocean Acidification? Retrieved from Fisheries and Oceans Canada: https://www.dfo-mpo.gc.ca/oceans/publications/soto-rceo/2012/page02-eng.html

Copyright © All Rights Reserved